Emission Lines Are Caused When an Electron Moves
Light is released as an electron moves to a lower energy state. The difference in emission lines are caused by the fact that helium has more electrons than hydrogen doesHydrogen has only 1 while helium has 2With more electrons being excited more spectral lines will be observed.

Formation Of Spectral Lines Astronomy
There are many possible electron transitions for.

. Furthermore what causes emission spectrum. The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a transition from a high energy state to a lower energy state. When the electron falls back to its ground level the light is emitted.
When excited an electron moves to a higher energy level or orbital. When the atoms absorb. What causes the appearance of lines in an emission spectrum.
What causes the appearance of lines in an emission spectrum. AnswerThe appearance of lines in an emission spectrum is caused by the fact that light is released as an electron moves to a lower energy state. An electron is released as light when it moves to a lower energy state.
When excited an electron moves to a higher energy level or orbital. Certain photons are absorbed when an electron moves from a higher to. What causes the dark lines that appear on an absorption spectrum.
An electron is released as light when it moves to a lower energy state. Emission lines are caused when an electron moves ____ energy. An absorption spectrum is characterized by dark lines or gaps while an emission spectrum typically shows colored lines.
Learn vocabulary terms and more with flashcards games and other study tools. The appearance of lines in an emission spectrum is caused by the fact that light is released as an electron moves to a lower energy state. Light is absorbed as an electron moves to a higher energy state.
Light is absorbed as an electron moves to a higher energy state. What causes the appearance of lines in an emission spectrum. When an atom gives an absorption spectrum it is because it has gained a higher energy level.
O The atom emits a photon when an electron moves from a higher to a lower energy level. He based this assumption on the fact that there are only a limited number of lines in the spectrum of the hydrogen atom and his belief that these lines were the result of light being emitted or absorbed as an electron moved from one orbit to another in the atom. This is called a line emission spectrum.
He postulated that the electron was restricted to certain orbits characterized by discrete energies. Start studying Chapter 7 Review. The atom emits a photon when an electron moves from a lower to a higher energy level.
Light is absorbed as an electron moves to a higher energy state. Light is released as an electron moves to a lower energy state. Atoms emit light when they are heated or excited at high energy levels.
See the answer See the answer See the answer done loading. The photon energy of the emitted photon is equal to the energy difference between the two states. Transitions between these allowed orbits result in the absorption or emission of photons.
So the appearance of lines in an emission spectrum is caused by. In contrast an emission spectrum results when an atom falls back to a lower level from an excited state with the release of energy. When the electron falls back to its ground level the light is emitted.
Absorption lines are caused when an electron moves _____ energy This problem has been solved. When an electron moves from a higher-energy orbit to a more stable one energy is. Then when those atoms that are unstable release energy they pass to a lower level and emit light.
O Light from an outside source shines on the atom. To calculate for helium a Rydberg constant of 594x10 15 s -1 is used. Light is released as an electron moves to a lower energy state.
An electron is released as light when it moves to a higher energy state. When the atoms absorb energy they get excited and reach a higher level of energy. The frequencies of light that an atom can emit are dependent on states the electrons can be in.
Emission spectrum is the spectrum of frequencies of electromagnetic radiation which are emitted due to an atom or molecule making a transition from a high energy state to a lower energy state. What causes the appearance of lines in an emission spectrum. Emission is the process of elements releasing different photons of color as their atoms return to their lower energy levels.
An electron is released as light when it moves to a higher energy state.
Why Does Hydrogen S Emission Spectrum Have Four Lines If Hydrogen Only Has One Electron Quora
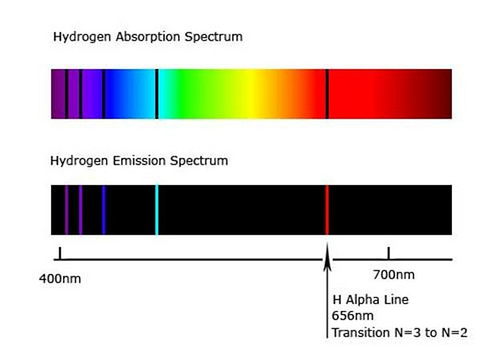
Absorption Spectrum Emission Spectrum Lines Article Khan Academy

No comments for "Emission Lines Are Caused When an Electron Moves"
Post a Comment